Turning yeast cells into labs for studying drivers of gene regulation
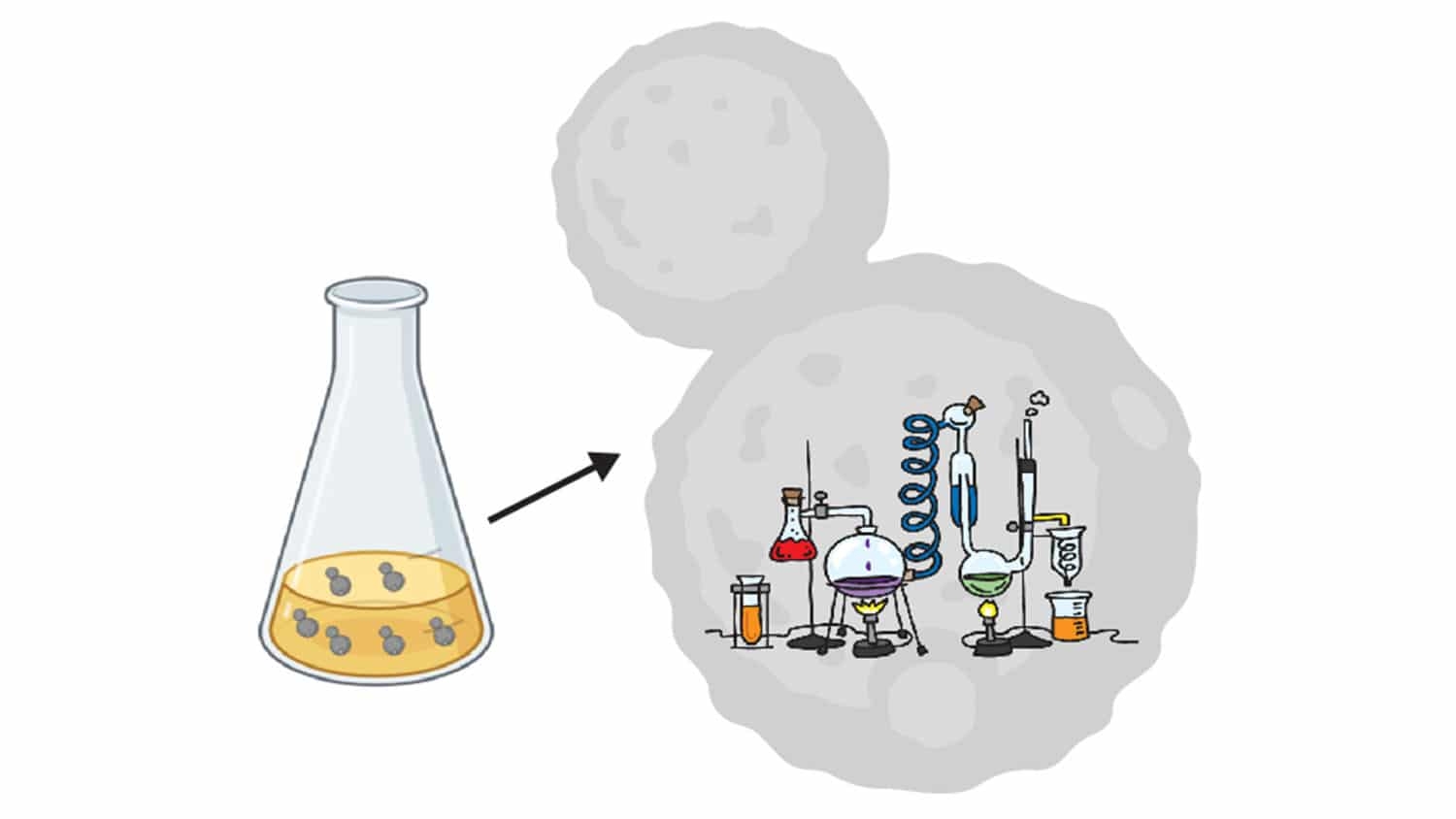
Researchers have developed a more efficient platform for studying proteins that play a key role in regulating gene expression. The approach uses engineered yeast cells to produce enzyme and histone proteins, conduct biochemical assays internally, and then display the results.
“Biomedical and biotech researchers are interested in the mechanisms that allow histones to regulate gene activity,” says Alison Waldman, first author of a paper on the work and a Ph.D. student at North Carolina State University. “But the conventional tools for histone research are unwieldy and slow. We wanted to develop something faster and less expensive – and we did.”
In complex organisms, chromosomes are largely made up of DNA and a group of proteins called histones. These histones are important for packing the DNA into chromosomes properly, but also play a role in regulating gene expression. In other words, they help determine when and how specific genes are turned on or off.
One of the features that makes histones challenging to study is that they often have chemical modifications that, alone or in combination, alter the role the histone plays in gene expression.
“Histones essentially serve as docking sites for other proteins that influence gene expression, and the chemical modifications we see on histones play a role in determining which proteins have access to a given gene,” says Balaji Rao, co-corresponding author of the paper and a professor of chemical and biomolecular engineering at NC State.
And to make matters more complicated, this is a dynamic process. A histone may have no modifications, it may retain a modification for the entire life of the cell, or modifications may be added and removed repeatedly. There is, in short, a lot going on. And enzymes are the catalysts responsible for all of those changes. Basically, enzymes are the mechanism for attaching or removing histone modifications.
So if you really want to understand what’s going on with histones, you have to understand the chemical modifications. But if you want to understand the chemical modifications, you need to understand which enzymes are present and what they are doing.
Conventional methods for understanding how enzymes modify a histone involve using one of two techniques. First, you could use chemical synthesis to create enzymes and histone proteins, then conduct an assay in a test tube to see what happens. Second, you could genetically engineer one bacteria to produce an enzyme and engineer other bacteria to produce histone proteins, then harvest the relevant proteins, purify them, then conduct an assay to see what happens.
“Our technique uses a genetically modified yeast cell to produce both the enzyme and the histone,” Waldman says. “The chemical modification takes place within the cell, and the resulting modified histone is sent to and displayed on the surface of the cell.”
“In other words, the yeast cell makes the relevant proteins, does the assay for you, and then displays the result on top,” says Albert Keung, co-corresponding author of the work and an assistant professor of chemical and biomolecular engineering at NC State.
The modified yeast platform is significantly faster than conventional techniques. For example, examining a single enzyme/histone pairing would take a couple days, instead of a week.
“But it’s easier to scale up than existing techniques, so you would save substantially more time if you were looking at a lot of proteins,” Keung says.
“In addition, there are some proteins that can’t be made using chemical synthesis, or that can’t be purified,” Rao says. “Our technique doesn’t require chemical synthesis or purification, which means we can look at proteins that were difficult or impossible to assay in the past.”
The researchers demonstrated the utility of the technique by having engineered yeast cells produce two types of histones and a well-studied enzyme called p300, which adds a specific acetyl group modification to histones.
“We’ve shown that our technique works,” Waldman says. “The next step is to begin expanding the modifications we’re looking at and scaling up the process.”
The paper, “Mapping the Residue Specificities of Epigenome Enzymes by Yeast Surface Display,” is published in the journal Cell Chemical Biology. The work was done with support from the National Science Foundation, under grant 1830910; and the National Institutes of Health, under grant R21EB023377.
-shipman-
Note to Editors: The study abstract follows.
“Mapping the Residue Specificities of Epigenome Enzymes by Yeast Surface Display”
Authors: Alison C. Waldman, Balaji M. Rao and Albert J. Keung, North Carolina State University
Published: June 28, Cell Chemical Biology
DOI: 10.1016/j.chembiol.2021.05.022
Abstract: Histone proteins are decorated with a combinatorially and numerically diverse set of biochemical modifications. Here we describe a versatile and scalable platform which enables efficient characterization of histone modifications without the need for recombinant protein production. As proof-of-concept, we first used this platform to rapidly profile the histone H3 and H4 residue writing specificities of the human histone acetyltransferase, p300. Subsequently, a large panel of commercially available antiacetylation antibodies for their specificities, identifying many suitable and unsuitable reagents. Further, enabled efficient mapping of the large binary crosstalk space between acetylated residues on histones H3 and H4 and uncovered previously unreported residue interdependencies affecting p300 activity. These results show that using yeast surface display to study histone modifications is a useful tool that can advance our understanding of chromatin biology by enabling efficient interrogation of the complexity of epigenome modifications.
This post was originally published in NC State News.
- Categories: