Nanocrystal ‘factory’ could revolutionize quantum dot manufacturing
A multidisciplinary team of NC State researchers has developed a microfluidic system for synthesizing perovskite quantum dots across the entire spectrum of visible light. The system drastically reduces manufacturing costs, can be tuned on demand to any color and allows for real-time process monitoring to ensure quality control.
Over the last two decades, colloidal semiconductor nanocrystals, known as quantum dots (QDs), have emerged as novel materials for applications ranging from biological sensing and imaging to LED displays and solar energy harvesting. The new system can be used to continuously manufacture high-quality QDs for use in these applications.
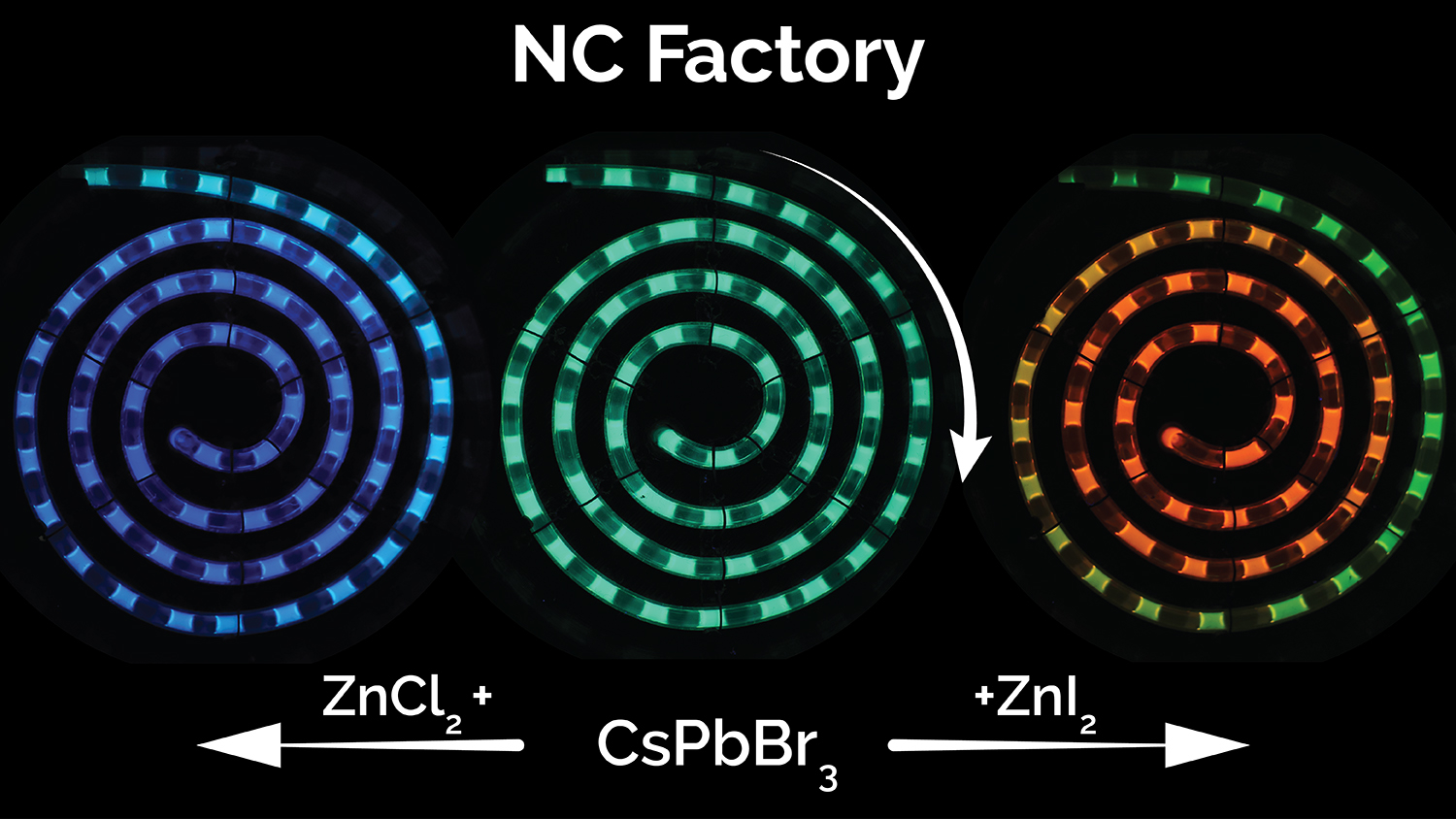
“We call this system the Nanocrystal (NC) Factory, and it builds on the NanoRobo microfluidic platform that we unveiled in 2017,” says Dr. Milad Abolhasani, an assistant professor in the Department of Chemical and Biomolecular Engineering (CBE) and corresponding author of a paper on the work.
“Not only can we create the QDs in any color using a continuous manufacturing approach, but the NC Factory system is highly modular,” Abolhasani says. “This means that, coupled with continuous process monitoring, the system allows modifications to be made as needed to eliminate the batch-to-batch variation that can be a significant problem for conventional QD manufacturing techniques. Additionally, the chemistry we have developed in this work allows the perovskite QD processing to take place at room temperature.”
The fluorescence color of QDs is a result of the chemical composition, the size, and the way the nanocrystals are processed. The original QD synthesis strategy utilized in the NanoRobo system allowed for the room temperature synthesis of green-emitting perovskite QDs, which are made using cesium lead bromide. NC Factory starts with cesium lead bromide perovskite quantum dots, but then introduces various halide salts to precisely tune their fluorescence color across the entire spectrum of visible light. Anions in these salts replace the bromine atoms in the green-emitting dots with either iodine atoms (to move toward the red end of the spectrum) or chlorine atoms (to move toward blue).
“Because the NC Factory can precisely control both chemical composition and processing parameters, it can be used to continuously manufacture perovskite quantum dots in any color with the highest quality,” Abolhasani says.
The NC Factory system consists of three “plug and play” modules. The researchers developed a pre-mixing module to expedite the mixing of halide salts and quantum dots, in order to improve product quality.
Co-first authors on the paper are Kameel Abdel-Latif and Robert Epps, Ph.D. students in CBE. The paper was co-authored by Corwin Kerr, an undergraduate student at NC State; Christopher Papa, a Ph.D. student in chemistry at NC State; and Dr. Felix Castellano, the Goodnight Innovation Distinguished Chair of Chemistry at NC State.
Return to contents or download the Fall/Winter 2019 NC State Engineering magazine (PDF, 2.3MB).
- Categories: