New system allows rapid response to heart attacks
Researchers in the UNC/NC State Joint Department of Biomedical Engineering (BME) have developed a drug-delivery system that allows rapid response to heart attacks without surgical intervention. In laboratory and animal testing, the system proved to be effective at dissolving clots, limiting long-term scarring to heart tissue and preserving more of the heart’s normal function.
“Our approach would allow health-care providers to begin treating heart attacks before a patient reaches a surgical suite, hopefully improving patient outcomes,” said Dr. Ashley Brown, corresponding author of a paper on the work and an assistant professor in BME. “And because we are able to target the blockage, we are able to use powerful drugs that may pose threats to other parts of the body; the targeting reduces the risk of unintended harms.”
Heart attacks occur when a thrombus — or clot — blocks a blood vessel in the heart. In order to treat heart attacks, doctors often perform surgery to introduce a catheter to the blood vessel, allowing them to physically break up or remove the thrombus. But not all patients have quick access to surgical care.
More damage can occur even after the blockage has been removed. That’s because the return of fresh blood to tissues that had been blocked off can cause damage of its own, called reperfusion injury.
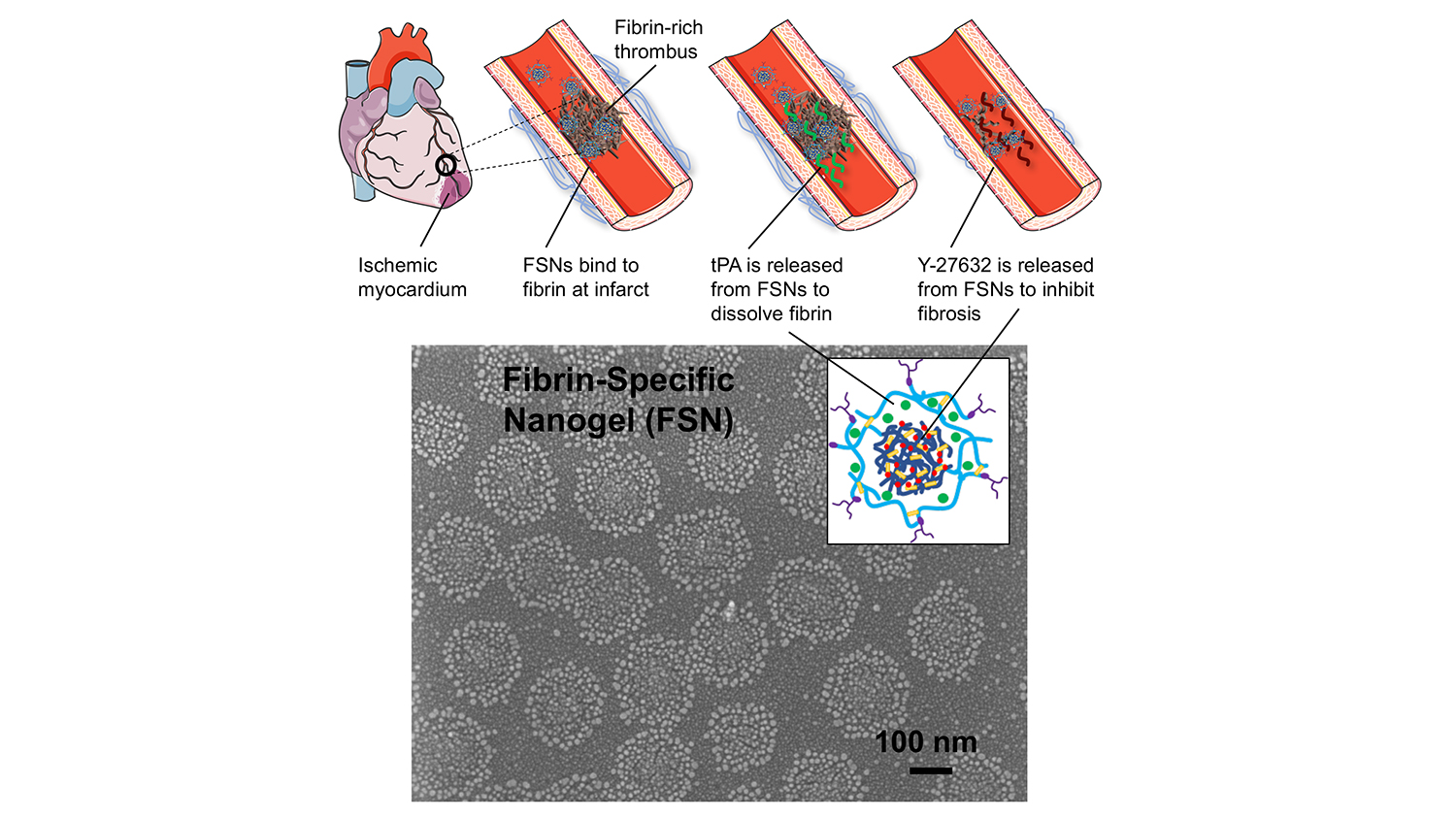
To address these problems, researchers have developed a solution that relies on porous nanogel spheres, about 250 nanometers in diameter, which target a thrombus and deliver a cocktail of two drugs: tPA and Y-27632.
A thrombus can be made of various substances, such as platelets or arterial plaques, but they all contain a substance called fibrin. So, to target blockages, each nanogel is coated with proteins that bind specifically to fibrin. In other words, when the nanogels reach a thrombus, they stick.
The tPA and Y-27632 are layered inside the nanosphere, with the tPA forming a shell that surrounds the Y-27632. As a result, the tPA leaks out first at the thrombus site, allowing it to do its job — which is to break down fibrin and dissolve the clot.
As the tPA is released, the Y-27632 escapes the nanogel. While the tPA targets the clot itself, the Y-27632 aims to limit the damage caused by reperfusion injury.
Co-lead authors are Emily Mihalko, a Ph.D. student in BME, and Ke Huang, a Ph.D. student in the College of Veterinary Medicine. The paper was co-authored by Dr. Ke Cheng, a professor of biomolecular sciences at NC State and a professor in BME, and by Dr. Erin Sproul, a former postdoctoral researcher in BME.
Return to contents or download the Spring/Summer 2019 NC State Engineering magazine (PDF, 13.7MB).
- Categories:


